About Us
Dedicated to be an exquisite specialized pharmaceutical research company
Beijing Wista Pharma Co., Ltd, founded in 2009, is located in Beijing Economic-Technological Development Area, committed to independent research and authorized production for chemical innovative drugs and high-end generic drugs.Our laboratory area is more than 2000 square meters, which has passed the environmental assessment, fire protection and safety assessment in Beijing, is equipped with a series of precise instruments, like, high performance liquid chromatograph, gas chromatograph, ion chromatography...
Technical Service
News
- Company News |
- Industry News

Our new formulation L-Ornithine-L-Aspartate Infusion for injection has been approved
Our new formulation L-Ornithine-L-Aspartate Infusion for injection( 5gm/10ml ) has been approved by China SFDA on 23th April 2020. The injection registration certificate...
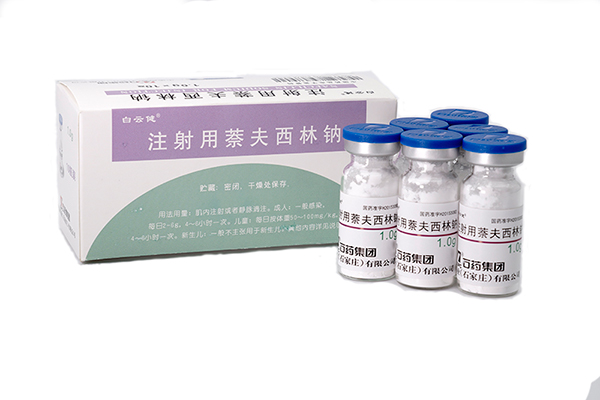
Nafcillin Sodium for injection has been approved by China SF
Our new formulation Nafcillin Sodium for injection (1.0gram per ampoule) has been approved by China SFDA on 4th May 2015. The API registration certificate no. is H2015308...

Olopatadine hydrochloride tablets has been approved by China SF
Our new formulation Olopatadine hydrochloride tablets (5mg ) has been approved by China SFDA on 31st May 2022. The tablets registration certificate No. is H20213892 and ...

 簡(jiǎn)體中文
簡(jiǎn)體中文





 86-10-67887855
86-10-67887855 Head Office:
Head Office: Marketing:
Marketing: Wista Pharmam
Wista Pharmam