-
 082024-05
082024-05Our new formulation L-Ornithine-L-Aspartate Infusion for injection has been approved
Our new formulation L-Ornithine-L-Aspartate Infusion for injection( 5gm/10ml ) has been approved by China SFDA on 23th April 2020. The injection registration certificate No. is H20203173. -
 082024-05
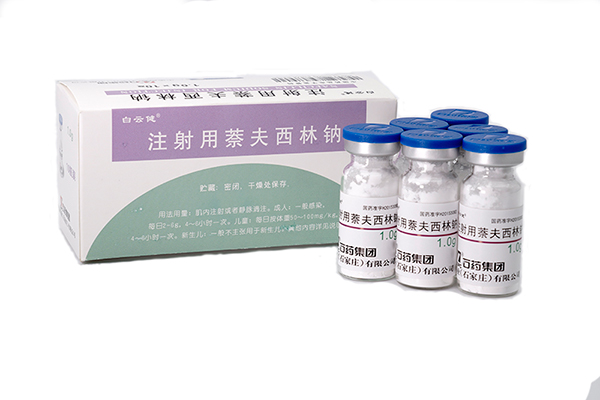
082024-05Nafcillin Sodium for injection has been approved by China SF
Our new formulation Nafcillin Sodium for injection (1.0gram per ampoule) has been approved by China SFDA on 4th May 2015. The API registration certificate no. is H20153081 and its injection registrati -
 082024-05
082024-05Olopatadine hydrochloride tablets has been approved by China SF
Our new formulation Olopatadine hydrochloride tablets (5mg ) has been approved by China SFDA on 31st May 2022. The tablets registration certificate No. is H20213892 and H20223350. -
 082024-05
082024-05Voriconazole tablets has been approved by China SF
Our new formulation Voriconazole tablets (50mg and 200mg) has been approved by China SFDA on 24th Nov. 2021. The tablets registration certificate No. is H20213892 and H20213893. -
 082024-05
082024-05Luliconazole and posaconazole are launched into market
The synthesis pilots of Luliconazole and posaconazole are successfully completed.The key intermediates are available to international market from July 2013.

 簡體中文
簡體中文
 86-10-67887855
86-10-67887855 Head Office:
Head Office: Marketing:
Marketing: Wista Pharmam
Wista Pharmam